
Like a broken record playing the same lousy lyrics over and over, every year sunscreen choices seem to be stuck on repeat with many products offering inferior sun protection and potentially unsafe ingredients.
EWG’s 2022 Guide to Sunscreens includes more than 1,850 sunscreens, and only about one in four products, or 500, meets our standards for adequate sun protection and avoids ingredients linked to known health harms. Products marketed for babies and kids do slightly better, on average, with one in three meeting our standards.
This year’s update for the first time includes a section detailing the widespread contamination of sunscreens, particularly sprays, with cancer-causing benzene. It’s another reminder of the need for stricter federal safety testing of all sunscreens.
Changes to U.S. regulation of sunscreen products and the ingredients approved for use in sunscreens have been years in the making, but they still haven’t reached the finish line. Over the past 25 years, new ingredients have been developed to provide stronger ultraviolet A, or UVA, protection, but none have been approved by the Food and Drug Administration, so they aren’t available to U.S. formulators. It’s important that consumers in this country have better products providing stronger protection from the sun, because UVA exposure is linked to increased melanoma risk, suppression of the immune system, DNA damage and premature aging, which can lead to skin cancer.
Last year, EWG scientists published the results of efficacy tests we conducted of U.S. sunscreens. Our findings showed UVA protection, and UV protection overall, to be inadequate – and less than consumers would expect, given the SPF value.
As many people eagerly await the return of time spent outdoors, sun safety is paramount. The sun’s rays can increase the risk of skin cancer, and a combination of proper sun-safe behaviors – such as wearing hats, clothing and sunglasses, avoiding the sun during its peak, and choosing a safe and effective sunscreen – is the best protection.
An EWG peer-reviewed study of sunscreen efficacy from late fall 2021 found that most sunscreens sold in the U.S. provide inadequate UVA protection from the sun’s harmful rays, compared to what the listed SPF suggests. This gives sunscreen users a false sense of safety. The article, published in the journal Photodermatology Photoimmunology and Photomedicine, includes results from tests of 51 sunscreen products. On average, products reduced the ultraviolet exposure by only half what would be expected, and only about a third of the products – just 18 of 51 – passed the UVA protection test required of sunscreens sold in Europe.
Although the SPF value is considered a good predictor of sunscreen effectiveness against UVB rays, the main cause of sunburn, most sunscreens we tested failed to show reliable protection. Poor sun protection can result in overexposure to UVA rays, which have been associated with long-term skin damage, wrinkles and an increased risk of skin cancer. We’re especially concerned by our finding that, on average, the UVA protection factor was only a quarter of the labeled SPF value.
Our sunscreen ranking system has been informed by our test results. It’s designed to provide the best recommendations for efficacy and low ingredient toxicity. Consumers should use our guide to find the products that best balance UVA and UVB protection.
As we have for more than 10 years, EWG continues to recommend that consumers avoid spray sunscreens, because of difficulty with proper application, potential ingredient toxicity and inhalation concerns.
Applying too thin a layer of sunscreen is one reason a sunscreen may not be effective, and this is especially a problem for aerosols. How thickly an aerosol sunscreen is applied dropped significantly in even a light breeze, according to tests by researchers from Australia’s Griffith University – enough that even application of as much as an entire container could fail to provide adequate sun protection. The Australian government now recommends consumers avoid aerosol sunscreens completely.


For the first time, this year’s Guide to Sunscreens includes a section on the detection of the carcinogenic contaminant benzene in spray sunscreens. Many products have been recalled because of widespread contamination with benzene.
Inhalation is yet another problem with aerosol sunscreens, which can be inhaled deep into the lungs, where they can cause irreversible damage. If the FDA proceeds with sunscreen regulations proposed in 2019 and again last year – which wouldn’t go into effect until next year at the earliest – manufacturers of all spray and powdered sunscreens will need to prove their products are safe. Test requirements are informed by FDA sample tests of aerosol products, which found that three of 14 tested sprays would not meet the agency’s proposed inhalation standard.
The FDA did not reveal which three products failed its test and should therefore be avoided.
Sunscreens are more heavily regulated than other cosmetic products, because of their classification as over-the-counter drugs. No new sunscreen ingredients have been approved for use since the 1990s, and there’s been little change to sunscreen regulation, though skin cancer rates have continued to climb and the importance of strong UVA protection has only increased.
The FDA’s proposed regulations require companies to provide more safety data about their ingredients, improve UVA protection, limit SPF values to 60+, ban products that combine sunscreen and bug repellent, improve labeling, and conduct additional tests for aerosol products.
These regulations are an improvement, but they don’t ensure that the only sunscreens on the market are those with adequate UVA protection that avoid the use of concerning ingredients.
One of the most important changes the FDA proposes is the recognition that there are just two ingredients we know enough about to be considered safe and effective – zinc oxide and titanium dioxide. The FDA proposed that 12 other common sunscreen active ingredients, including oxybenzone, undergo more safety and efficacy tests before it makes any determinations about them. These ingredients are used in just over half the sunscreens in this year’s guide. The FDA requested the same data for them that it requires for sunscreen ingredients used in other countries that companies want approved for use in the U.S.
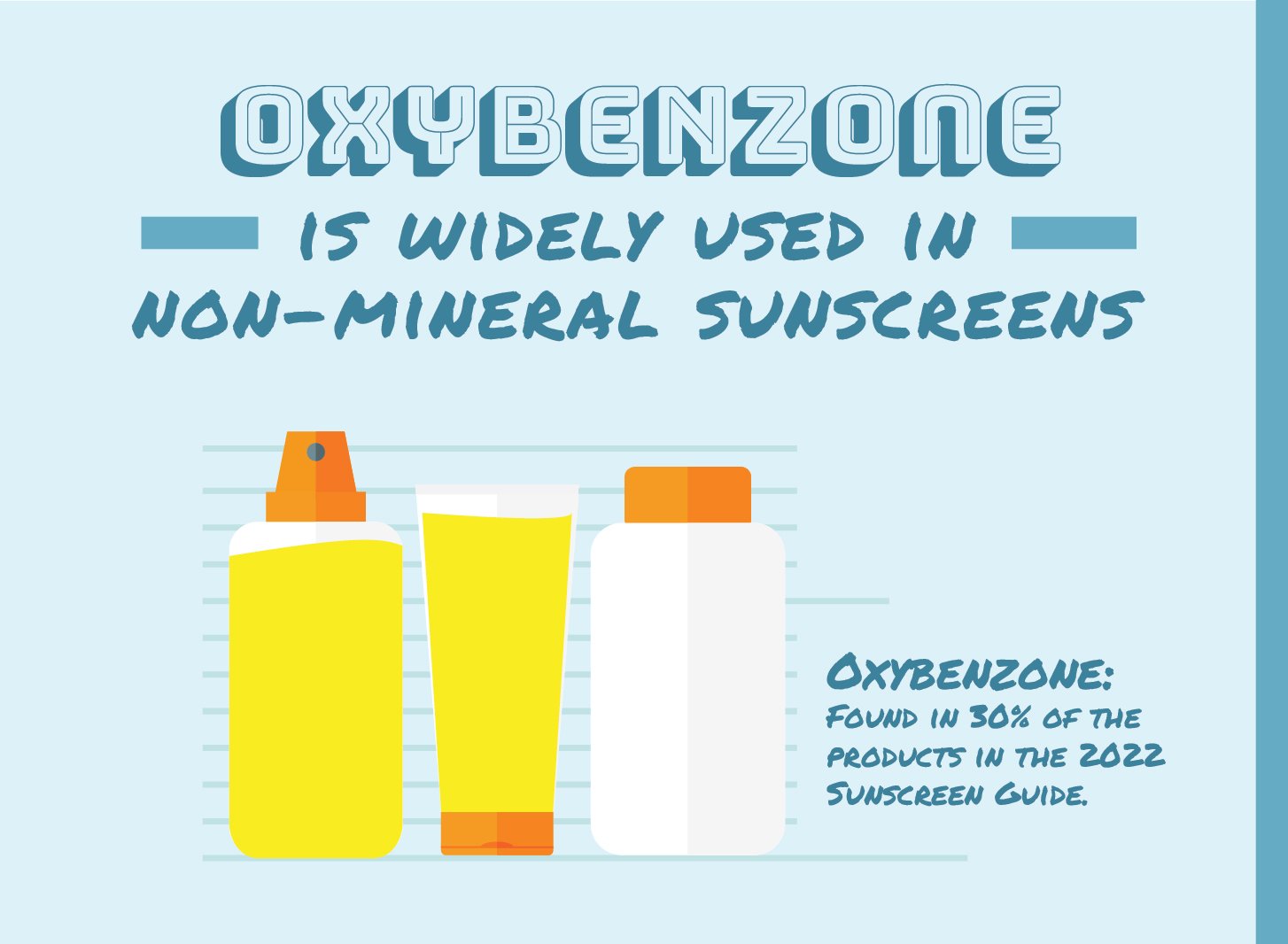
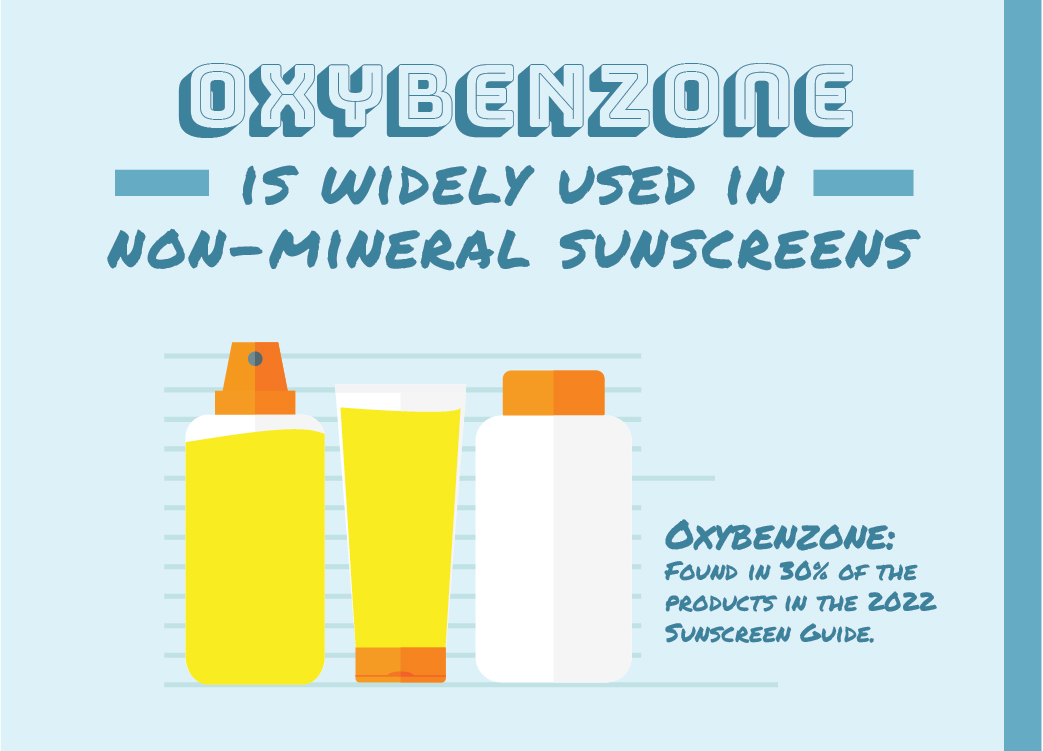
Oxybenzone is a common active ingredient in sunscreen, found in about 15 percent of the products assessed this year, including about a third of non-mineral options. EWG first called on the FDA to study its safety in 2008, citing significant evidence showing that it easily penetrates the skin and could potentially disrupt the hormone system.
In 2019, the FDA said current data did not justify classifying oxybenzone as safe and effective. Even though the chemical is one of the most studied sunscreen ingredients, the FDA requested more data, because test results had raised health concerns and oxybenzone has been found in human breast milk, amniotic fluid, urine and blood.
The FDA has requested more research on oxybenzone, especially on:
The FDA notes children may be more vulnerable than adults to harm from oxybenzone “because of the potential for higher absorption and bioaccumulation.”
Four studies published in 2020, after the initial FDA proposal, support the agency’s earlier finding – that oxybenzone can act as an endocrine disruptor and may increase the risk of breast cancer and endometriosis.
The number of non-mineral products in our guide that contain oxybenzone has dropped by half since 2019, which suggests that despite the FDA’s failure to restrict the use of oxybenzone, the market is responding to safety concerns on its own.
Higher SPF values have not been shown to provide greater clinical benefit than those with lower values and may provide users with a false sense of security. To guard against misconceptions about high-SPF sunscreen efficacy, in 2019 and again last year, the agency proposed limiting SPF claims to 60+. The FDA has emphasized the need for this SPF upper limit, along with proposed changes to the broad-spectrum test, to ensure sunscreens provide more UVA protection.
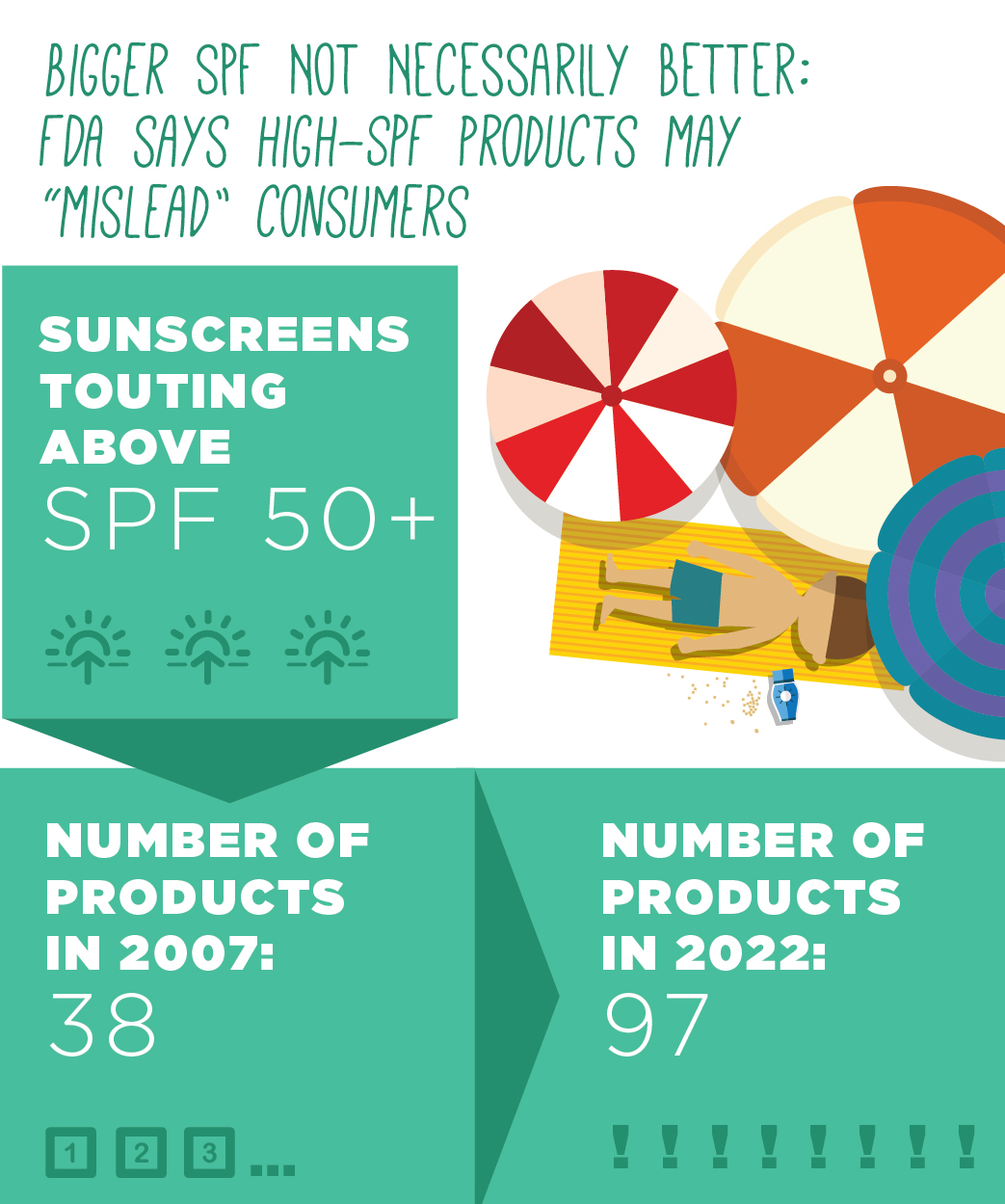
In 2011, the FDA recommended an SPF cap of 50+, but that proposal was never finalized. EWG sees the more recent recommendation as a step backward. To justify its revision, the FDA cites only studies of sunscreens with active ingredients barred from use in the U.S. Based on the research that the FDA reviewed, there was no clear evidence that allowing higher SPF products will be beneficial to consumers.
Products with high SPF values are both very popular and widely available. More than one in 20 of the sunscreens we reviewed claim an SPF above 50+. EWG recommends consumers avoid products labeled SPF 50+ and higher.

A new section of the 2022 Guide to Sunscreens details the widespread contamination of sunscreens – particularly aerosols – with the cancer-causing chemical benzene. (Read our research here.) A study last year raised concerns after finding benzophenone, another carcinogenic contaminant, widespread in sunscreens that used octocrylene. Other recent studies of cosmetic products have found contamination from PFAS chemicals and even asbestos.
The FDA should immediately require that sunscreen products be tested for harmful contaminants like benzene. Consumers shouldn’t be exposed to harmful or cancer-causing chemicals in the products they use every day.
Last fall EWG published an analysis of how melanin levels in the skin change both overall risk of skin cancer and where it is most likely to occur. An understanding of how melanin levels change the risk of UV damage, the risk of skin cancer, and the role melanin plays in vitamin D levels is essential to the most accurate advice. EWG will continue to research this topic.
In the meantime, avoid sunburns – a clear marker of too much harmful UV exposure – and follow our sun safety tips.
EWG provides information on sunscreen products from the published scientific literature, to supplement incomplete data available from companies and the government. The ratings indicate both efficacy and the relative level of concern posed by exposure to the ingredients in this product - not the product itself - compared to other sunscreens. The ratings reflect potential health hazards but do not account for the level of exposure or individual susceptibility, factors which determine actual health risks, if any. Methodology | Privacy Policy | Terms & Conditions
